Comparative Assessment of Genetic Variability Realised in Doubled Haploids Induced from F1 and F2 Plants for Response to Fusarium Stalk Rot and Yield Traits in Maize (Zea mays L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Basic Plant Material
2.2. Development of Experimental Material
2.3. Field Layout
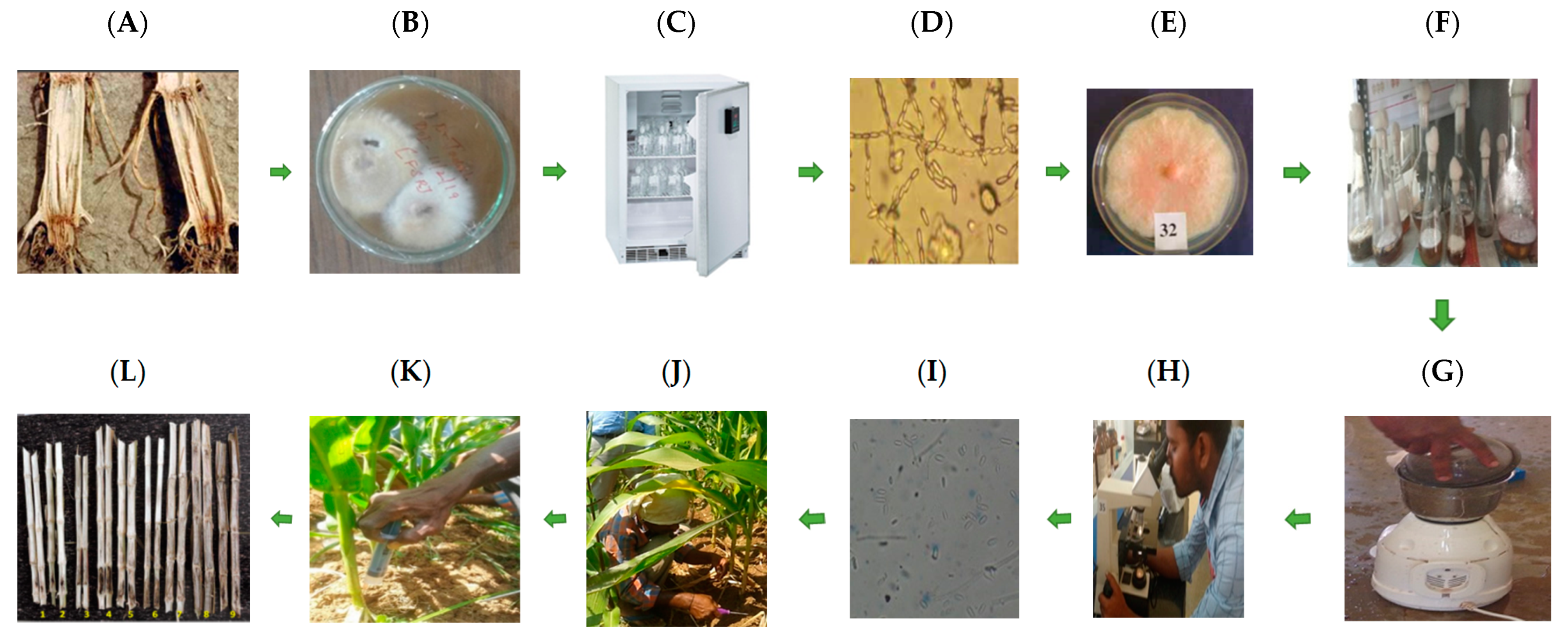
2.4. Screening for Resistance to Fusarium Stalk Rot
2.5. Isolation and Mass Multiplication of F. verticilloides Pathogen
2.6. Phenotyping of DH Lines for Their Response to FSR
2.7. Comparative Assessment of Genetic Variability for Quantitative Traits
2.8. Assessment of Combining-Ability Variance of DH Lines Derived from F1 and F2 Generations of the Cross VL1043 × CM212
2.8.1. Crossing Program
2.8.2. Evaluation of Crosses
2.9. Statistical Analysis
2.9.1. Analysis of DH Lines
2.9.2. Comparative Assessment of Genetic Variability of DH Lines in Terms of Quantitative Traits
2.9.3. Correlation Analysis
3. Results
3.1. Classification of DHs into Different Disease-Response Groups
3.2. Classification of F2 Plants into Different Disease-Response Groups
3.3. Genetic Variability Parameters and Components of Variance
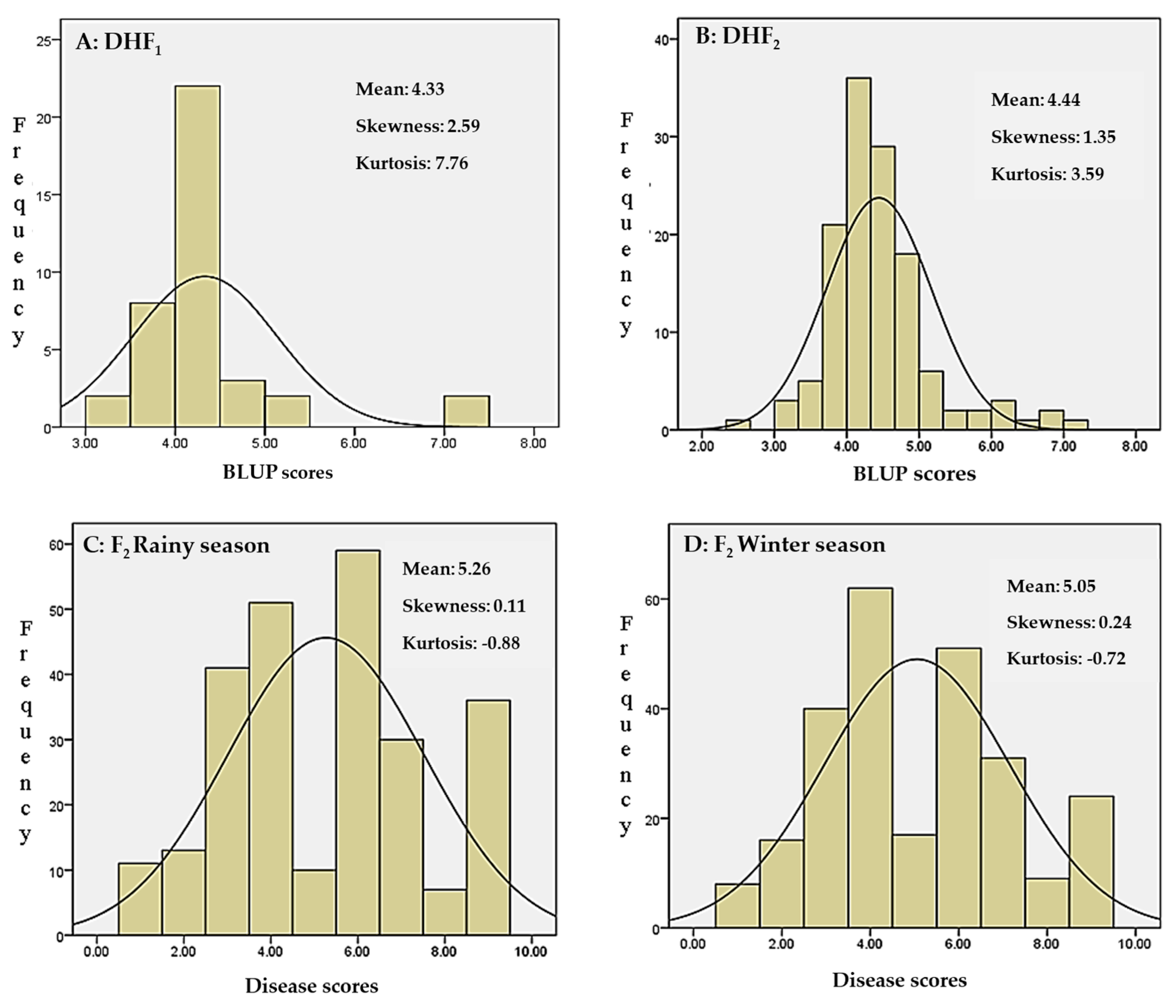
3.4. Population Distribution
3.5. Comparative Assessment of Genetic Variability Released from Doubled-Haploid Lines Produced from F1 and F2 Generations in Terms of Yield-Related Traits
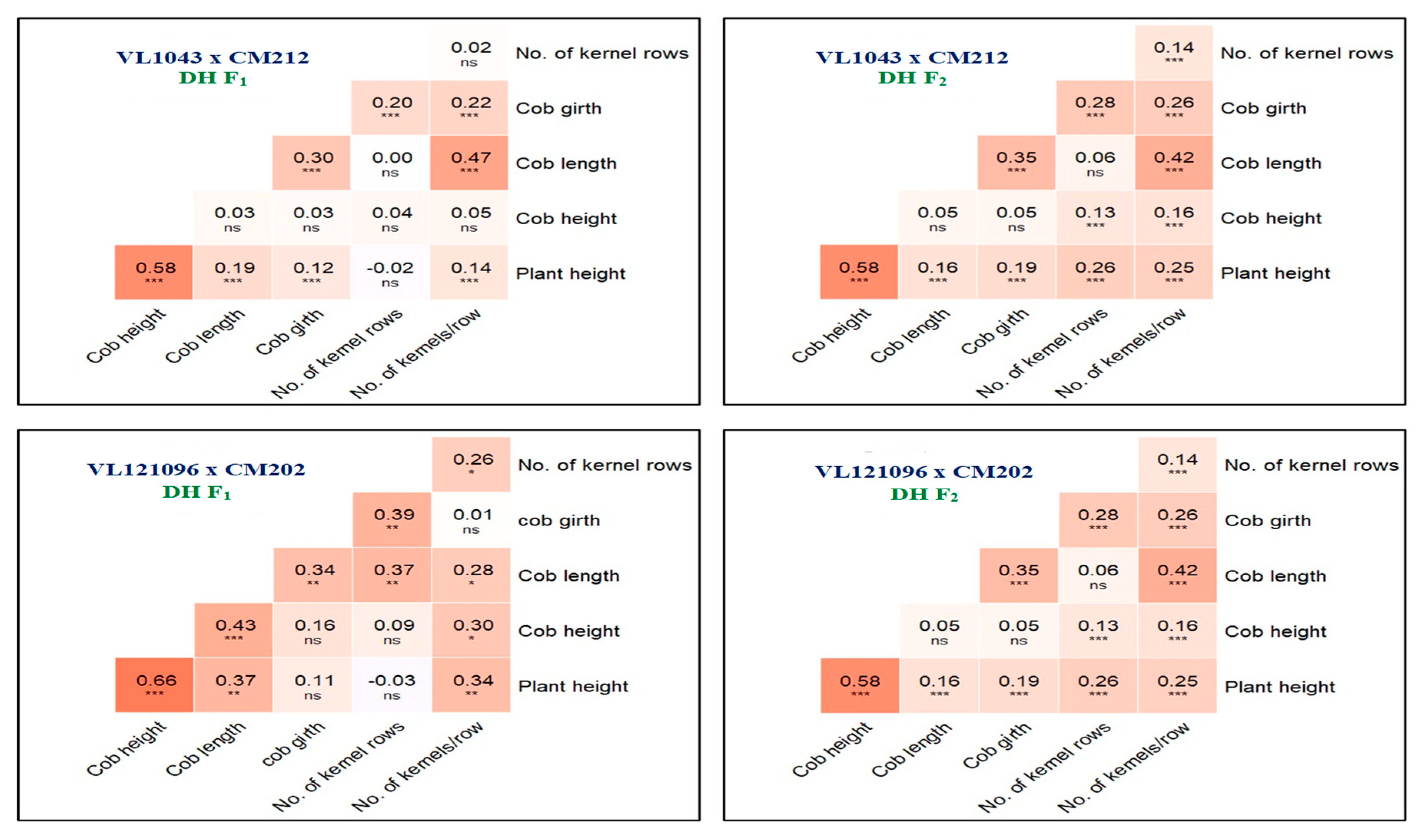
3.6. Characteristic Association among Productivity Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hooda, K.S.; Sekhar, J.C.; Karjagi, C.G.; Kumar, S. Identifying sources of multiple disease resistance in maize. Maize J. 2012, 1, 82–84. [Google Scholar]
- Desai, S.; Hegde, R.K.; Desai, S. A preliminary survey of incidence of stalk rot complex of maize in two districts of Karnataka. Indian Phytopath. 1991, 43, 575–576. [Google Scholar]
- Kumar, M.; Lal, H.C.; Jha, M.M. Assessment of yield loss due to post-flowering stalk rots in maize. J. Appl. Biol. 1998, 8, 90–92. [Google Scholar]
- Harlapur, S.I.; Wali, M.C.; Prashanth, M.; Shakuntala, N.M. Assessment of yield losses in maize due to charcoal rot in Ghataporabha Left Bank Cannal (GLBC) command area of Karnataka. Karnataka J. Agric. Sci. 2002, 15, 590–591. [Google Scholar]
- Cook, R.J. The incidence of stalk rot (Fusarium spp.) on maize hybrids and its effect on yield of maize in Britain. Ann. Appl. Biol. 1978, 88, 23–30. [Google Scholar] [CrossRef]
- Couto, E.G.D.O.; Cury, M.N.; E Souza, M.B.; Granato, S.C.; Vidotti, M.S.; Garbuglio, D.D.; Crossa, J.; Burgueño, J.; Fritsche-Neto, R. Effect of F1 and F2 generations on genetic variability and working steps of doubled haploid production in maize. PLoS ONE 2019, 14, e0224631. [Google Scholar] [CrossRef]
- Chaikam, V.; Lopez, L.A.; Martinez, L.; Burgueño, J.; Boddupalli, P.M. Identification of in vivo induced maternal haploids in maize using seedling traits. Euphytica 2017, 213, 177. [Google Scholar] [CrossRef] [Green Version]
- Chaikam, V.; Gowda, M.; Martinez, L.; Ochieng, J.; Omar, H.A.; Prasanna, B. Improving the Efficiency of Colchicine-Based Chromosomal Doubling of Maize Haploids. Plants 2020, 9, 459. [Google Scholar] [CrossRef] [Green Version]
- Bernardo, R. Should maize doubled haploids be induced among F1 or F2 plants? Theor. Appl. Genet. 2009, 119, 255–262. [Google Scholar] [CrossRef]
- Rober, F.; Gordillo, G.A.; Geiger, H.H. In vivo haploid induction in maize-performance of new inducers and significance of doubled haploid lines in hybrid breeding. Maydica 2005, 50, 275–283. [Google Scholar]
- Riggs, T.J.; Snape, J.W. Effects of linkage and interaction in a comparison of theoretical populations derived by diploidized haploid and single seed descent methods. Theor. Appl. Genet. 1977, 49, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Jannink, J.-L.; Abadie, T.E. Inbreeding Method Effects on Genetic Mean, Variance, and Structure of Recurrent Selection Populations. Crop Sci. 1999, 39, 988–997. [Google Scholar] [CrossRef]
- Mowers, R.P.; Foster, D.J. Genetic Variance Estimates for Maize Yield, Grain Moisture, and Stalk Lodging for Doubled-Haploid and Conventional Selfed-Line Hybrids. Plants 2020, 9, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Archana, R.; Lohithaswa, H.C.; Uma, M.S.; Shivakumar, K.V.; Sanathkumar, V.B.; Pavan, R. Genetic analysis of Fusarium stalk rot resistance in maize (Zea mays L.). J. Pharmacogn. Phytochem. 2019, SP1, 58–61. [Google Scholar]
- Chaikam, V.; Molenaar, W.; Melchinger, A.E.; Boddupalli, P.M. Doubled haploid technology for line development in maize: Technical advances and prospects. Theor. Appl. Genet. 2019, 132, 3227–3243. [Google Scholar] [CrossRef] [Green Version]
- Available online: www.jiffypot.com (accessed on 10 October 2020).
- Federer, W.T. Augmented Designs with One-Way Elimination of Heterogeneity. Biometrics 1961, 17, 447. [Google Scholar] [CrossRef]
- Anonymous. Indian Institute of Maize Research Bulletin; 2012. [Google Scholar]
- Payak, M.M.; Sharma, R.C. Disease rating scale in maize in India. In Techniques of Scoring for Resistance to Important Diseases of Maize; New Delhi: All India Coordinated Maize Improvement Project; IARI: New Delhi, India, 1983; pp. 1–5. [Google Scholar]
- Hooker, A.L. Association of resistance to several seedling, root, stalk, and ear diseases of corn. Phytopathology 1956, 46, 379–384. [Google Scholar]
- Schonfeld, P.; Werner, H.J. Beitragr zur teorie und anwendung linearer modelle. In Okonomische Progress, entscheidungsund gleichgewichtsmodelle; Krelle, W., Ed.; VCH Verlagsgesellschaft: Weinheim, Germany, 1986; pp. 251–262. [Google Scholar]
- Patterson, H.D.; Thompson, R. Recovery of inter-block information when block sizes are unequal. Biometrika 1971, 58, 545–554. [Google Scholar] [CrossRef]
- Federer, W.T.; Wolfinger, R.D. SAS code for recovering intereffect information in experiments with incomplete block and lattice rectangle designs. Agron. J. 1998, 90, 545–551. [Google Scholar] [CrossRef]
- Lush, J.L. Heritability of quantitative characters in farm animals. Hereditas 1945, 35, 356–375. [Google Scholar] [CrossRef]
- Burton, G.W.; DeVane, E.H. Estimating Heritability in Tall Fescue (Festuca arundinacea) from Replicated Clonal Material. Agron. J. 1953, 45, 478–481. [Google Scholar] [CrossRef]
- Robinson, H.F.; Comstock, R.E.; Harvey, P.H. Estimates of Heritability and the Degree of Dominance in Corn. Agron. J. 1949, 41, 353–359. [Google Scholar] [CrossRef]
- Johnson, H.W.; Robinson, H.F.; Comstock, R.E. Estimates of Genetic and Environmental Variability in Soybeans. Agron. J. 1955, 47, 314–318. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 5th ed.; Iowa State University Press: Ames, IA, USA, 1994. [Google Scholar]
- Dewey, D.R.; Lu, K.H. A Correlation and Path-Coefficient Analysis of Components of Crested Wheatgrass Seed Production. Agron. J. 1959, 51, 515–518. [Google Scholar] [CrossRef]
- Prigge, V.; Xu, X.; Li, L.; Babu, R.; Chen, S.; Atlin, G.N.; Melchinger, A.E. New insights into the genetics of in vivo induction of maternal haploids, the backbone of doubled haploid technology in maize. Genetics 2012, 190, 781–793. [Google Scholar] [CrossRef] [Green Version]
- Dunwell, J.M. Haploids in flowering plants: Origins and exploitation. Plant Biotechnol. J. 2010, 8, 377–424. [Google Scholar] [CrossRef]
- Boopathi, N.M. Genetic Mapping and Marker Assisted Selection: Basics, Practice and Benefits; Springer: New York, NY, USA, 2012; pp. 1–293. [Google Scholar] [CrossRef]
- Khulbe, R.K.; Pattanayak, A.; Kant, L.; Bisht, G.S.; Pant, M.C.; Pandey, V.; Kapil, R.; Mishra, N.C. Doubled haploid production in maize under Sub-montane Himalayan conditions using R1-nj-based haploid inducer TAILP1. Indian J. Genet. Plant Breed. 2020, 80, 261–266. [Google Scholar] [CrossRef]
- Nair, S.K.; Chaikam, V.; Gowda, M.; Hindu, V.; Melchinger, A.E.; Boddupalli, P.M. Genetic dissection of maternal influence on in vivo haploid induction in maize. Crop J. 2020, 8, 287–298. [Google Scholar] [CrossRef]
- Singh, B.D.; Singh, A.K. Marker-Assisted Plant Breeding: Principles and Practices; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–16. [Google Scholar] [CrossRef]
- Rotarenco, V.A.; Chalyk, S.T.; Jacota, A.; Eder, J. Breeding effect of selection at the level of haploid sporophyte in maize. Bull. Acad. Sci. Mold. 2007, 1, 92–98. [Google Scholar]
- Geiger, H.H.; Gordillo, G.A.; Koch, S. Genetic Correlations among Haploids, Doubled Haploids, and Testcrosses in Maize. Crop Sci. 2013, 53, 2313–2320. [Google Scholar] [CrossRef]
- Sleper, J.A.; Bernardo, R. Recombination and genetic variance among maize doubled haploids induced from F1 and F2 plants. Theor. Appl. Genet. 2016, 129, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
- Melchinger, A.E.; Schmidt, W.; Geiger, H.H. Comparison of Testcrosses Produced from F 2 and First Backcross Populations in Maize. Crop Sci. 1988, 28, 743–749. [Google Scholar] [CrossRef]
- Schnicker, B.J. Comparison of Genetic Variances in F2 and Backcross Populations of Maize; Iowa State University Digital Repository: Ames, IA, USA, 1992; Available online: http://lib.dr.iastate.edu (accessed on 25 June 2019). [CrossRef]
- Choo, T.M. Doubled haploids for estimating additive epistatic genetic variances in self-pollinating crops. Can. J. Genet. Cytol. 1980, 22, 125–127. [Google Scholar] [CrossRef]
- Weir, B.S.; Cockerham, C.C.; Reynolds, J. The effects of linkage and linkage disequilibrium on the covariances of noninbred relatives. Heredity 1980, 45, 351–359. [Google Scholar] [CrossRef] [Green Version]
- Snape, J.W.; Simpson, E. The genetical expectations of doubled haploid lines derived from different filial generations. Theor. Appl. Genet. 1981, 60, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Ogunniyan, D.; Olakojo, S. Genetic variation, heritability, genetic advance and agronomic character association of yellow elite inbred lines of maize (Zea mays L.). Niger. J. Genet. 2014, 28, 24–28. [Google Scholar] [CrossRef] [Green Version]
- Choo, T.M.; Reinbergs, E. Analyses of Skewness and Kurtosis for Detecting Gene Interaction in a Doubled Haploid Population. Crop Sci. 1982, 22, 231–235. [Google Scholar] [CrossRef]
- Snape, J.; Riggs, T.J. Genetical consequences of single seed descent in the breeding of self-pollinating crops. Heredity 1975, 35, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Choo, T.M.; Christie, B.R.; Reinbergs, E. Doubled haploids for estimating genetic variances and a scheme for population improvement in self-pollinating crops. Theor. Appl. Genet. 1979, 54, 267–271. [Google Scholar] [CrossRef]
- Robson, D.S. Applications of the k 4 Statistic to Genetic Variance Component Analyses. Biometrics 1956, 12, 433. [Google Scholar] [CrossRef]
- Covarrubias-Prieto, J.; Hallauer, A.R.; Lamkey, K.R. Intermating F2 populations of maize. Genetika 1989, 21, 111–126. [Google Scholar]
- Arbelbide, M.; Bernardo, R. Random Mating before Selfing in Maize BC1 Populations. Crop Sci. 2004, 44, 401–404. [Google Scholar] [CrossRef]
- Reddy, V.R.; Jabeen, F.; Sudarshan, M.R.; Rao, A.S. Studies on genetic variability, heritability, correlation and path analysis in maize (Zea mays L.) over locations. Int. J. Appl. Biol. Pharm. 2012, 4, 195–199. [Google Scholar]
- Lingaiah, N.; Kumar, G.P.; Venkanna, V. Character association and path analysis for yield contributing and physiological parameters for grain yield in maize (Zea mays L.). Int. J. Pure Appl. Biosci. 2014, 2, 118–121. [Google Scholar]
- Supraja, V.; Sowmya, H.C.; Kuchanur, P.H.; Arunkumar, B.; Kisan, B. Genetic Variability and Character Association Studies in Maize (Zea mays L.) Inbred Lines. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 646–656. [Google Scholar] [CrossRef]
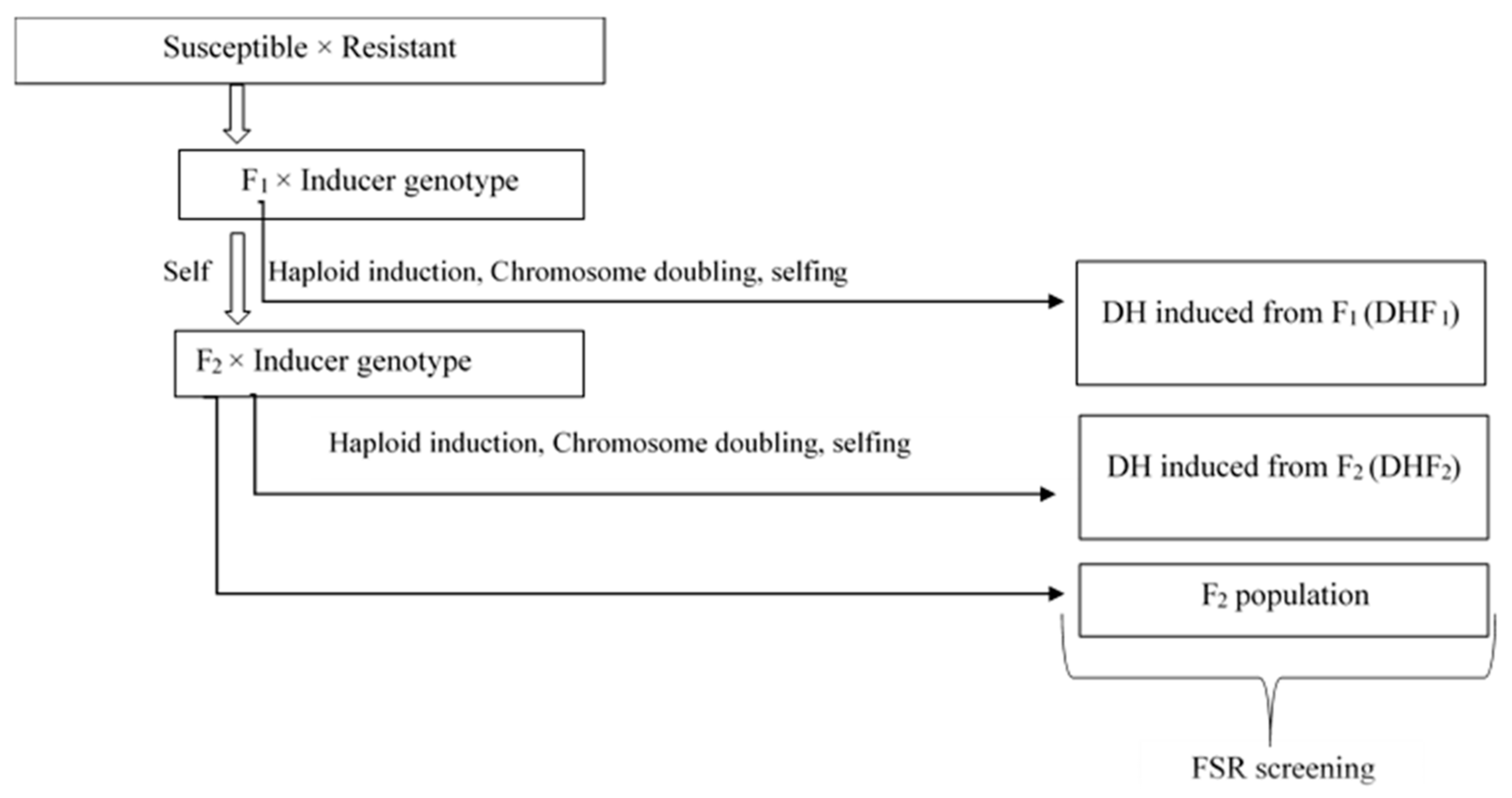

| Cross | No. of Doubled-Haploid Lines Induced from F1 (DHF1) | No. of Doubled-Haploid Lines Induced from F2 (DHF2) | No. of Individual F2 Plants |
|---|---|---|---|
| VL1043 × CM212 | 339 | 329 | 220 |
| VL121096 × CM202 | 39 | 130 | 258 |
| Score | Symptoms | Disease Reaction |
|---|---|---|
| 1 | Healthy or slight discoloration at the site of inoculation | Highly Resistant |
| 2 | Up to 50% of the inoculated internode is discolored | Resistant |
| 3 | 51–75% of the inoculated internode is discolored | Moderately Resistant |
| 4 | 76–100% of the inoculated internode is discolored | Moderately Susceptible |
| 5 | Less than 50% discoloration of the adjacent internode | Susceptible |
| 6 | More than 50% discoloration of the adjacent internode | Highly Susceptible |
| 7 | Discoloration of three internodes | Highly Susceptible |
| 8 | Discoloration of four internodes | Highly Susceptible |
| 9 | Discoloration of five or more internodes and premature death of a plant | Highly Susceptible |
| Source | VL1043 × CM212 | VL121096 × CM202 | ||||||
|---|---|---|---|---|---|---|---|---|
| DHF1 | DHF2 | DHF1 | DHF2 | |||||
| Blocks | 12 | 0.05 ns | 12 | 0.02 ns | 5 | 0.05 ns | 5 | 0.038 ns |
| Seasons | 1 | 0.39 * | 1 | 3.51 *** | 1 | 0.33 ns | 1 | 0.399 ns |
| Checks | 1 | 253.35 *** | 1 | 223.02 *** | 1 | 96.80 *** | 1 | 96.00 *** |
| Doubled haploids | 338 | 0.69 *** | 328 | 0.89 *** | 38 | 1.15 *** | 129 | 0.967 *** |
| Seasons vs. Doubled haploids | 339 | 0.21 *** | 329 | 0.22 *** | 39 | 0.22 * | 130 | 0.164 ns |
| Error | 37 | 0.08 | 37 | 0.06 | 16 | 0.09 | 16 | 0.128 |
| Disease Score | Disease Response | VL1043 × CM212 | VL121096 × CM202 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Doubled-Haploid Lines | Number of Individual F2 Plants | No. of Doubled-Haploid Lines | Number of Individual F2 Plants | ||||||
| DHF1 | DHF2 | Rainy Season 2019 | Winter Season 2019–2020 | DHF1 | DHF2 | Rainy Season 2019 | Winter Season 2019–2020 | ||
| 1 | Highly resistant | 00 | 00 | 02 | 02 | 00 | 00 | 11 | 08 |
| 2 | Resistant | 00 | 01 | 03 | 03 | 00 | 01 | 13 | 16 |
| 3 | Moderately resistant | 59 | 61 | 23 | 22 | 10 | 29 | 41 | 40 |
| 4 | Moderately susceptible | 235 | 220 | 108 | 103 | 25 | 83 | 51 | 62 |
| 5 | Susceptible | 35 | 31 | 41 | 41 | 02 | 10 | 10 | 17 |
| >6–9 | Highly susceptible | 10 | 16 | 43 | 49 | 02 | 07 | 132 | 115 |
| Population size | 339 | 329 | 220 | 220 | 39 | 130 | 258 | 258 | |
| Genetic Parameters | Genetic Estimates (BLUP Scores) | |||
|---|---|---|---|---|
| VL1043 × CM212 | VL121096 × CM202 | |||
| DHF1 | DHF2 | DHF1 | DHF2 | |
| Mean | 4.44 | 4.45 | 4.33 | 4.44 |
| Range | 3.51–7.89 | 2.79–8.48 | 3.20–7.28 | 2.42–7.31 |
| Standardized range | 0.99 | 1.28 | 0.94 | 1.10 |
| Genetic variance | 0.28 | 0.41 | 0.55 | 0.40 |
| Residual variance | 0.08 | 0.06 | 0.09 | 0.13 |
| Phenotypic coefficient of variation | 13.51 | 15.50 | 18.47 | 16.39 |
| Genotypic coefficient of variation | 11.92 | 14.48 | 17.12 | 14.24 |
| Heritability | 0.78 | 0.87 | 0.86 | 0.75 |
| Genetic advance | 0.96 | 1.24 | 1.42 | 1.13 |
| Genetic advance as per cent mean | 21.65 | 27.89 | 32.69 | 25.49 |
| Skewness | 2.20 | 1.81 | 2.59 | 1.35 |
| Kurtosis | 7.30 | 5.81 | 7.76 | 3.59 |
| Genetic Parameters | VL1043 × CM212 | VL121096 × CM202 | ||
|---|---|---|---|---|
| Rainy Season 2019 | Winter Season 2019–2020 | Rainy Season 2019 | Winter Season 2019–2020 | |
| Mean | 4.51 | 4.60 | 5.26 | 5.05 |
| Range | 1–9 | 1–9 | 1–9 | 1–9 |
| Standardized range | 1.77 | 1.74 | 1.52 | 1.58 |
| Genetic variance | 0.78 | 0.63 | 4.39 | 3.41 |
| Residual variance | 0.71 | 1.03 | 0.70 | 1.00 |
| Phenotypic coefficient of variation | 27.07 | 27.98 | 42.86 | 41.55 |
| Genotypic coefficient of variation | 19.61 | 17.24 | 39.82 | 36.52 |
| Heritability | 0.52 | 0.38 | 0.86 | 0.77 |
| Genetic advance | 1.32 | 1.01 | 4.01 | 3.34 |
| Genetic advance as per cent mean | 29.25 | 21.89 | 76.19 | 66.15 |
| Skewness | 0.71 | 0.68 | 0.11 | 0.24 |
| Kurtosis | 1.32 | 0.93 | −0.88 | −0.72 |
| Characters | VL1043 × CM212 | VL121096 × CM202 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | t—Calculated for Comparing Means | VG | Standard Deviation | Mean | t—Calculated for Comparing Means | VG | Standard Deviation | |||||||
| DHF1 | DHF2 | DHF1 | DHF2 | DHF1 | DHF2 | DHF1 | DHF2 | DHF1 | DHF2 | DHF1 | DHF2 | |||
| Plant height (cm) | 129.76 | 121.26 | 43.66 | 317.4 | 363.21 | 17.82 | 19.06 | 116.42 | 120 | 6.27 | 263.06 | 488.41 | 16.22 | 22.10 |
| Ear height (cm) | 57.48 | 45.34 | 97.02 | 192.68 | 167.1 | 13.88 | 12.93 | 51.2 | 49.3 | 4.79 | 105.31 | 183.32 | 10.26 | 13.54 |
| Ear length (cm) | 11.54 | 13.15 | 26.94 | 3.63 | 3.64 | 1.91 | 1.91 | 11.36 | 13.02 | 11.73 | 3.82 | 3.82 | 1.95 | 1.95 |
| Ear circumference (cm) | 11.11 | 11.99 | 17.81 | 1.01 | 1.13 | 1.00 | 1.06 | 11.41 | 12.21 | 6.09 | 0.95 | 1.7 | 0.97 | 1.30 |
| Kernel rows per cob | 12.47 | 12.36 | 2.15 | 1.9 | 1.72 | 1.38 | 1.31 | 12.07 | 12.99 | 7.38 | 3.55 | 3.05 | 1.88 | 1.75 |
| Kernels per row | 21.52 | 20.91 | 2.89 | 27.81 | 14.45 | 5.27 | 3.80 | 16.38 | 21.3 | 15.21 | 30.93 | 22.07 | 5.56 | 4.70 |
| table t-value = 1.96 (p = 0.05) | table t-value = 1.96 (p = 0.05) | |||||||||||||
| Crosses Derived from DHF1 Lines | Crosses Derived from DHF2 Lines | |
|---|---|---|
| Mean | 0.758 | 0.757 |
| Standard deviation | 0.299 | 0.217 |
| σ2A | 0.151 | 0.133 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Showkath Babu, B.M.; Lohithaswa, H.C.; Triveni, G.; Mallikarjuna, M.G.; Mallikarjuna, N.; Balasundara, D.C.; Anand, P. Comparative Assessment of Genetic Variability Realised in Doubled Haploids Induced from F1 and F2 Plants for Response to Fusarium Stalk Rot and Yield Traits in Maize (Zea mays L.). Agronomy 2023, 13, 100. https://doi.org/10.3390/agronomy13010100
Showkath Babu BM, Lohithaswa HC, Triveni G, Mallikarjuna MG, Mallikarjuna N, Balasundara DC, Anand P. Comparative Assessment of Genetic Variability Realised in Doubled Haploids Induced from F1 and F2 Plants for Response to Fusarium Stalk Rot and Yield Traits in Maize (Zea mays L.). Agronomy. 2023; 13(1):100. https://doi.org/10.3390/agronomy13010100
Chicago/Turabian StyleShowkath Babu, Budensab Mamtazbi, Hirenallur Chandappa Lohithaswa, Gangadharaswamy Triveni, Mallana Gowdra Mallikarjuna, Nanjundappa Mallikarjuna, Devanagondi C. Balasundara, and Pandravada Anand. 2023. "Comparative Assessment of Genetic Variability Realised in Doubled Haploids Induced from F1 and F2 Plants for Response to Fusarium Stalk Rot and Yield Traits in Maize (Zea mays L.)" Agronomy 13, no. 1: 100. https://doi.org/10.3390/agronomy13010100





